Description
The CareStart COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of the nucleocapsid protein antigen from SARS-CoV-2 in nasopharyngeal or anterior nasal swab specimens directly collected from individuals who are either suspected of COVID-19 by their healthcare provider within the first five days of symptom onset or from individuals without symptoms or other epidemiological reasons to suspect COVID-19 when tested twice over two or three days with at least 24 hours and no more than 48 hours between tests.
Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests. This test is authorized for use at the Point of Care (POC), i.e., inpatient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation.
NOTE: For Serial Screening of asymptomatic individuals. The serial screening indication is only applied to products manufactured by Access Bio Inc. after April 12, 2021. The product batches listed in the Official Notification Letter (click to see) should not be marketed or used for POC serial screening purposes.
AMA CPT Codes
FDA EUA Letter
Package Insert
Fact Sheet for Healthcare Providers











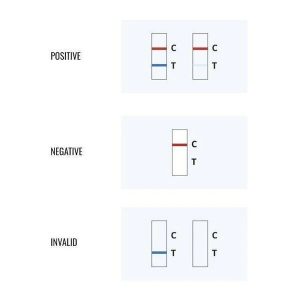
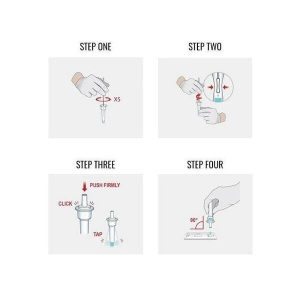









Kaden Arabic (verified owner) –
Good quality.
John (verified owner) –
Good quality.